As an FDA registered contract manufacturer of Over-The-Counter (OTC) cosmetic products, we play a crucial role in helping you, our valued clients, develop safe and compliant finished goods. What follows is intended as an educational device to illustrate the primary steps in developing a cosmetic product containing a monographed OTC drug, and how that might differ from a more typical cosmetic product. This is not meant to serve as a policy statement or to be interpreted as a replacement for regulatory expertise, or as legal advice. We’re writing this today because it is a murky, often misunderstood topic that could use a little sunlight. It’s something no one ever talks about, which is exactly why we’re doing so.
The primary difference between a cosmetic and an OTC cosmetic is their intended use. A cosmetic is defined as something “rubbed, poured, sprinkled, or sprayed on, introduced into, or otherwise applied to the human body…for cleansing, beautifying, promoting attractiveness, or altering the appearance.” (FD&C Act, Sec 201(i)). Interestingly (especially if you make soap) soap is excluded – but that’s for another blog. OTC Drug Products, on the other hand, are “articles intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease” and “articles (other than food) intended to affect the structure or any function of the body of man or other animals”. (FD&C Act, Sec 201 (g)(1)) Click Here for hyperlink to FDA site.
In order to discuss the process of developing an OTC product we have to make a few fundamental assumptions:
- You have a brand that has identified a skin condition that your consumers are afflicted with; and
- You have identified an “active ingredient” that is effective in the treatment, diagnosis, cure, mitigation, or prevention of the aforementioned skin condition.
- You do not intend to develop a new drug, thus the active ingredient will be found on an existing Monograph or FDA Administrative Order.
- For the purposes of this blog, we’re not talking about Homeopathic drugs, or Medical Devices, or SPF Products.
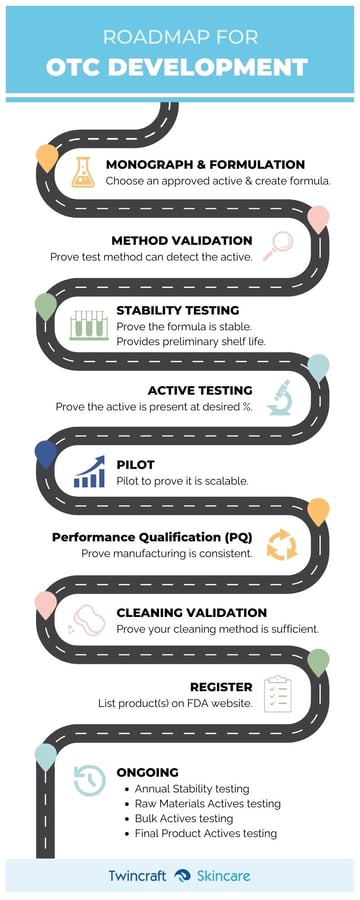 Our story begins where an ordinary cosmetic development typically ends. By now we have been through an iterative process of refining a formula texture, selecting a package, and finally we are at the Formula Approval stage. For most cosmetics all that remains is to conduct stability testing, package compatibility testing, preservative challenge testing and to scale-up through a piloting process. For a drug product, we’ll still conduct each of these activities, but there is far more work to do.
Our story begins where an ordinary cosmetic development typically ends. By now we have been through an iterative process of refining a formula texture, selecting a package, and finally we are at the Formula Approval stage. For most cosmetics all that remains is to conduct stability testing, package compatibility testing, preservative challenge testing and to scale-up through a piloting process. For a drug product, we’ll still conduct each of these activities, but there is far more work to do.
In an OTC, as with a cosmetic product, stability testing determines the shelf-life of the product. Stability Testing for OTC products seeks to understand the duration for which the product is safe to use, and how long it retains its therapeutic value. This testing is done multiple times during the development process to confirm the results are repeatable, and the testing is conducted at least once annually thereafter. Testing is done in both real-time and on an accelerated basis. Pricing for this testing varies depending upon the active ingredient chosen. During development, since it will be conducted multiple times, there is a one-time charge associated which is meant to cover the entirety of the Stability testing required for launch. Post-launch, the testing is a once per year charge which is usually not included in the cost per unit.
Actives Testing verifies the identity and potency of active ingredients to substantiate label claims and to ensure the product lives up to regulatory standards for safety and efficacy. Basically, we need to test to make sure that an active ingredient is present in the formula at the concentration desired. Actives Testing is performed on the active raw material, the formulated bulk product, and again on finished goods. From a cost standpoint, post-launch, we include the actives testing in the cost of the finished goods. During development, since there is so much more Actives Testing involved in the various process steps, we include the Actives Testing as part of Performance Qualification (PQ), something we’ll discuss in detail separately.
To conduct Actives Testing, you must work with an accredited lab to ensure that there is a proven method for detecting an active ingredient and its concentration. This process is known as Method Validation, and it usually takes about 4-8 weeks to complete. Method Validation confirms that the analytical procedure used by the lab can provide useful, legitimate data. This is an integral part of any good analytical practice. Method Validation is a one-time fee incurred during the development process and is quoted at the onset of the development after consultation with an outside laboratory.
As with any cosmetic, we will conduct a Pilot as part of the development of an OTC cosmetic. A pilot is a small-scale production run and is an essential part of any development process. OTC pilots must be conducted at a prescribed proportion (> 10%) of the intended production batch size, so thoughtful consideration must go into the Pilot Plan. OTC pilots must be filled into the final primary package, but the packaging need not be decorated as long as the resulting product is not used for sale. Pilots filled into decorated packaging can occasionally yield saleable goods if they meet all criteria for doing so, namely that the artwork is final and approved, and that there is sufficient passing Stability Testing results to justify the expiration date. Pilot goods must be destroyed if supporting data is not available to yield saleable goods. The benefits of a pilot include all the benefits of a typical cosmetic pilot (www.twincraft.com/twincraft-skincare-blog/the-true-purpose-of-a-pilot-run), but requires significantly more testing, including Active Testing. The cost of a pilot varies widely depending on the cost of the formula itself, the batch size, filling variables, and testing requirements.
We’re not quite done yet. Before we can market saleable goods, it must be demonstrated that the manufacturing process employed for production of an OTC product is suitable for the intended task, yielding effective and repeatable results. This includes qualifying the manufacturing equipment, the ancillary systems, and validating the process. This function is known as Performance Qualification (PQ). PQ is the most time-consuming and costly part of the OTC development process because it must be repeated multiple times. A protocol is written in advance outlining the very specific process PQ will undergo. A well written PQ Protocol will include (but not be limited to) the formula number, active ingredient and concentration, batch size, batch tank and filling equipment, order of ingredient addition (the formula making instructions, or FMI), any processing variables such as temperature and mixing speeds, cleaning process, sampling and analysis plan, acceptance criteria, etc. PQ includes many of the topics already covered in this article such as Pilots, Actives Testing, and Stability Testing, but at its most basic core, it consists of conducting and testing no fewer than three batches.
Regarding the equipment, we must prove that it can be cleaned sufficiently to prevent cross-contamination of the active with whatever product is batched or filled next on the same equipment. The Cleaning Validation confirms that the cleaning process employed for a specific active ingredient in or on a specific piece or pieces of equipment is suitable for its intended task. Results from the Cleaning Validation can be used to judge the quality, reliability and consistency of the process.
Lastly, your OTC product must be registered with the FDA. Registration must be done not later than 30-days after the product hits the market, but it can be done sooner if we have approved artwork and an approved formula. Each “flavor” variant, fill size variant, and labeling variation (distribution market, for example) is a new finished good that must be registered.
So, that’s it. We’ve touched on all the major milestones that must be achieved in the development of an OTC cosmetic product. For the more experienced product developers on the brand side, this process may be routine, but for those of you encountering OTC cosmetics for the first time, the process, the jargon, and the regulatory complexity can feel daunting. We are here to assist with your questions, and to guide you through the process from ideation to launch.





